Chemistry Assignment Help with Types of Electrodes
9.12 Types of Electrodes
In an electrochemical cell, there are two electrodes, positive and negative. Each electrode constitutes a half cell or a single electrode. Although a number of electrodes are possible but the more important of these electrodes are grouped into the following types:
(i) Metal-metal ion electrodes
(ii) Metal-metal insoluble salt electrodes
(iii) Metal-amalgam electrodes
(iv) Gas-ion electrodes (v) Oxidation-reduction or redox electrodes.
(v) Oxidation-reduction or redox electrodes.
We shall denote these electrodes as right hand ones, i.e., the electrode reactions would correspond to reductions.
(i) Metal-metal ion electrodes : These electrodes consist of a pure metal (M) in contact with a solution of its cation (Mn+). For example, a silver rod immersed in a solution of Ag+ ions or copper rod in copper sulphate solution. The electrode is represented as Mn+ and the electrode reaction can be written as
Mn+ (a) + ne– 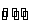 M
M
Since the electrode is reversible to the metal cation, its electrode potential can be expressed as
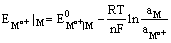
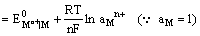
and depends on the activity of the metal cation in the solution.
(ii) Metal-metal insoluble salt electrodes: Such electrodes are important and are frequently used in electrochemical works. These consist of a metal (M) covered by a layer of sparingly soluble salt (MX) immersed in a solution containing a common anion (X–). Examples of such electrodes are mercury-mercurous sulphate in contact with a solution of potassium sulphate or a silver wire coated with silver chloride immersed in potassium chloride solution. These electrodes are represented as
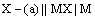
The overall electrode reaction is
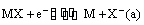
and the electrode potential is given by
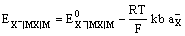
The electrode is reversible to the anion of the sparingly soluble salt, X–.
(iii) Metal amalgam electrodes: Sometimes when the metal is highly reactive, it is more convenient to use the metal in the form of amalgams. The activity of the metal is lowered by dilution with mercury. These electrodes are generally set up by placing the metal-amalgam in contact with a solution of metal ion. These electrodes are represented as
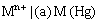
and the electrode reaction is
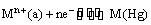
The electrode potential is given by
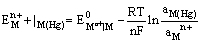
(iv) Gas-ion electrodes: A gas electrode consists of an inert metal usually gold or platinum immersed in a solution containing ions to which the gas is reversible. A current of pure gas is continuously bubbled through the solution. The inert metal electrode does not participate in the electrode reaction but simply helps in making electrical contact. The gas (X2) electrode is denoted as
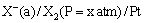
The electrode reaction can be written as
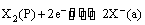
(v) Oxidation-reduction or redox electrodes: These are electrodes in which the emf arises from the presence of ions of a substance in two different oxidation states. These electrodes are set up by dipping an inert metal like gold or platinum into a solution containing ions in two different oxidation states of the substance. For example, a platinum wire immersed in a solution of ferrous and ferric ions or stannous and stannic ions constitutes a redox electrode. These electrodes are represented as
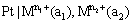
and the electrode reaction is
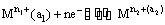
where 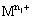 is the higher oxidation state and
is the higher oxidation state and 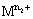 is the lower oxidation state.
is the lower oxidation state.
Email Based Assignment Help in Types of Electrodes
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Types of Electrodes in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Types of Electrodes. Our service is focused on: time delivery, superior quality, creativity and originality.
To submit chemistry Types of Electrodes assignment click here.


