Chemistry Assignment Help with Electrode Potential
9.13 Electrode Potential
The potential of any electrode is the potential difference between it and the electrolyte surrounding the electrode. The electrode potential depends upon the nature of the metal, concentration of the metallic ions in solution and the temperature of the solution. When the ions are at unit activity and the temperature is 25°C (298K), the potential difference is called a standard electrode potential (SEO). The potential of a single electrode cannot be determined but the potential difference between the two electrodes can be accurately measured. Normal hydrogen electrode (NHE), also called standard hydrogen electrode (SHE), is the standard reference electrode 1. Thus the standard electrode potential of a metal may be defined as the potential difference in volts developed in a cell consisting of two electrodes, the pure metal in contact with a molar solution of one of its ions and the normal hydrogen electrode. Electrode Potential—Factors Affecting It
Electrode Potential—Factors Affecting It
![]()
Electrode potential of oxidation half-cell M/Mn+ (aq) with reaction
![]() is
is
![]()
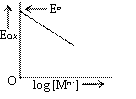
Eox depends on
(i) [Mn+], concentration of Ionic species
If [Mn+] increases, Eox decreases (figure)
We can say electrode is reversible with respect to Mn+
(ii) Temperature T
If T increases at constant [Mn+], Eox decreases.
Electrode potential of reduction half-cell Mn+ (aq) / M with reaction
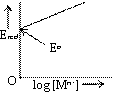
![]() is
is

Email Based Assignment Help in Electrode Potential
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Electrode Potential in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Electrode Potential. Our service is focused on: time delivery, superior quality, creativity and originality.
To submit chemistry Electrode Potential assignment click here.


