Chemistry Assignment Help with Electrolysis
9.8 Electrolysis
The process of decomposition of an electrolyte on passing an electric current through its aqueous solution or in the fused state is called electrolysis. An electrolyte (AB) when dissolved in water or when melted dissociates to produce corresponding ions (e.g., A+ and B–). When the circuit is completed by closing key, following reactions occur at the two electrodes.
(a) The cations move towards cathode. On reaching cathode, they gain electrons (supplied by battery) and thus become neutral atoms
(At cathode) A+ + e– —® A (Reduction)
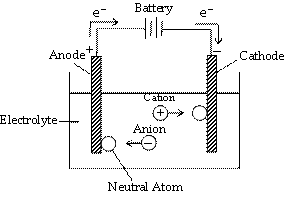

(b) The anions move towards anode and on reaching the anode they lose electrons and converted to neutral atoms.
(At anode) B– —® B + e– (Oxidation)
Thus electrons from the source (battery) enter the solution (where they are taken up by cations) at the cathode and leave the solution at the anode. As a result, flow of electricity continues along with the liberation of ions at the electrodes.

Email Based Assignment Help in Electrolysis
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Electrolysis in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Electrolysis. Our service is focused on: time delivery, superior quality, creativity and originality.
To submit chemistry Electrolysis assignment click here.


