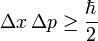Uncertainty Principle
Uncertainty Principle - Physics | Online Chemistry Help
According to the quantum mechanics in physics, the position and momentum of a moving electron can not be determined with great accuracy. When an electron is considered as a wave, it is, however, not possible to know the exact location of the electron on the wave as it (wave) is extending throughout a region of space. Thus, The question arises - If an electron is exhibiting dual nature (wave and particle), is it possible to know the exact position of the electron in space at some given instant?
The answer to this question is given by Heisenberg in 1927, which states that “It is impossible to determine simultaneously the position and momentum of the electron with any desired accuracy.”
In other words - "The position and momentum of a particle cannot be simultaneously measured with arbitrarily high precision. There is a minimum for the product of the uncertainties of these two measurements. There is likewise a minimum for the product of the uncertainties of the energy and time."
Uncertainty principleThe above definition is known as Heisenberg's uncertainty principle.
The German physicist Werner Heisenberg (1901-1976) received the Nobel Prize in physics in 1932 for his great contribution in nuclear physics and quantum theory. Werner Heisenberg formulated the uncertainty principle at Niels Bohr's institute in Copenhagen, while working on the mathematical foundations of quantum mechanics. The paper ( published by Werner Heisenberg in 1927 ) on the uncertainty relation is his most important contribution to physics.
Mathematical Relation
Heisenberg's principle can be stated mathematically as
Dp ´ Dx ³ h/4p …(1)
Where Dp is the uncertainty in the determination of the momentum and Dx, the uncertainty in the determination of position. Equation (1) is known as Heisenberg's equation which can be stated in words as, “the product of uncertainty in the simultaneous determination of the position and momentum of a particle is equal or greater than the Planck's constant.”
Alternative statement
Sometimes instead of measuring position and momentum of the system, its energy E and the time t for which it remains in that energy state are measured. In these cases the uncertainty in measurement is represented as
DE ´ Dt ³ h/4p …(2)i.e. If the time for which the system remains in a particular energy state is short, then its energy will be more defined and for longer stay in a state, the energy will not be so well defined. The uncertainty principle is now regarded as a fundamental principle of nature.

Customer Support Center (24/7 technical support)
Assignmenthelp.net's Help Services – We are ready to help students around the clock on Physics and Chemistry. We will offer quality educational services to our customers through E-mail (support@assignmenthelp.net), Live Chat and Phone. We provide plagiarism free Physics/Chemistry Courses to the world-wide students and also offer free download facilities of physics and chemistry tutorials as well. Expert online physics and chemistry tutors at assignmenthelp.net provide all sorts of help regarding your problems in physics (atomic/quantum theory, quantum mechanics, and uncertainty principle ) and chemistry through e-mail and live chat.
To submit Chemistry/Physics CoursesClick here