Spectrum of Hydrogen Atom Assignment Help
The spectrum of each element is unique to each element or compound. As a result, the spectrum of each substance can be used to identify that substance. (Note that the Rydberg equation tells us only the spectrum of hydrogen.)
The atoms of all elements emit radiation when energized in an electric current, and as do all molecules of all compounds. For a hydrogen atom, for example, these changes in energy must correspond to the amounts of energy which the electrons inside the atom can gain or lose.
A potential difference is applied across the electrodes of the discharge tube containing hydrogen gas at very low pressure. This results in the emission of bluish light from the discharge tube. The light is then passed through a prism and allowed to fall on the screen producing line spectrum.
The hydrogen spectrum contains various isolated sharp lines with dark area in-between. The wavelength of these lines varies from ultraviolet region to infrared region of the electromagnetic radiations. These spectral lines were classified into six groups which were named after the name of their discoverer.
These spectral lines are as follows: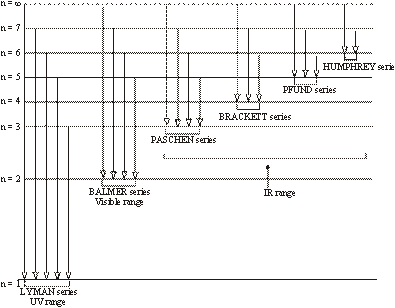

- (i). Lyman series – U.V. Region
- (ii). Balmer series – Visible Region
- (iii). Paschen series – I.R. Region
- (iv). Brackett series – I.R. Region
- (v). Pfund series – I.R. Region
- (vi). Humphrey series – I. R. Region
If an electrons jumps from higher orbit (E2) to lower orbit (E1), then energy released is given by
DE = E2 – E1![]()
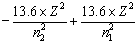
or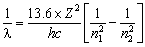
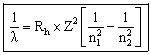
Where
![]() = wavelength (nm)
= wavelength (nm)
Rh = Rydberg's constant = 1.09678 x 10-2 nm-1
n1 = the lower energy level
n2 = the higher energy level

Customer Support Center
Assignmenthelp.net's Help Services – ready to help students around the clock (24/7 technical support) Live Support by Phone, Chat or E-mail (support@assignmenthelp.net). We provide plagiarism free Physics/Chemistry Courses to the students and also offer free download facilities of physics and chemistry tutorials as well. Expert online physics and chemistry tutors at assignmenthelp.net provide all sorts of help regarding your problems in physics (spectrum of hydrogen atom, atomic/quantum theory) and chemistry through e-mail and live chat.
To submit Physics/Chemistry Courses Click here


