Photoelectric Effect Assignment Help
Photoelectric Effect
In early 20th century physics two thoughts stand out as being totally revolutionary: Relativity and Quantum theory. World knows Albert Einstein is best known for his theory of relativity; he also played a major role in developing quantum theory, and it was his contribution to quantum theory - explaining the photoelectric effect - which won Nobel Prize in 1921.
Einstein's paper explaining this effect was one of the earliest applications of quantum theory and a major step in its establishment. To explain this effect one has to consider that light behaves like a stream of particles called photons.
"The photoelectric effect is a process whereby light falling on a surface knocks electrons out of the surface. Each electron is ejected by a single photon or light quantum striking the surface." Electrons emitted in this manner may be referred to as "photoelectrons". First observed by Heinrich Hertz in 1887, the phenomenon is also known as the "Hertz effect".
In the quantum theory, the frequency ( f ) of the light determines the energy ( E ) of the photons in that light beam. E = hf
Photoelectric Effect Assignment Help By Online Tutoring and Guided Sessions at AssignmentHelp.Net
Where h is Planck's constant [h = 6.626069 x 10-34 Joule seconds]. The energy of the emitted electron is given by the energy of the photon minus the energy needed to release the electron from the surface. It thus depends on the frequency of light falling on the surface, but not on its intensity.
Later, it was observed by J.J. Thomson and P. Lenard that when a beam of light of suitable wavelength or frequency is allowed to fall on the surface of a metal, the electrons are emitted (or ejected) from the surface of the metal. This phenomenon of emission of electrons is known as “photoelectric effect”. The electrons emitted are known as photoelectrons.
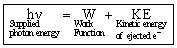
It was observed that this effect depends upon the frequency of the radiation as well as the nature of the metal surface. Let us consider a metallic surface struck by a photon beam having energy hn (n = the frequency of striking photon). A certain amount of this supplied energy is consumed by the bonded electron to get free from the metal-surface. The amount of energy thus required is known as work function (f).
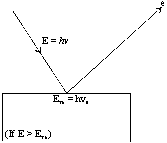
The remaining the supplied energy is converted into the kinetic energy of the free electrons. So,
 When the energy of the striking photon is equal to the work function then, frequency of the striking photon is known as Threshold Frequency(n0).
When the energy of the striking photon is equal to the work function then, frequency of the striking photon is known as Threshold Frequency(n0).
So,
hn0=W
By equation (1) and (2)
hn = hn0 + KE
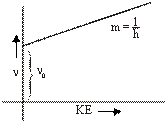
or 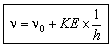
Our Customer Service Support Center
Assignmenthelp.net's Services – ready to help students around the clock (24/7 technical support) Live Supports by Phone, Chat or E-mail support@assignmenthelp.net. We provide plagiarism free Physics/Chemistry Courses to the students and also offer free download facilities of physics and chemistry tutorials as well. Expert online chemistry and physics tutors at Assignmenthelp.net provide all sorts of help regarding your problems in physics (photoelectric effect, atomic/quantum theory) and chemistry through e-mail and live chat.

To submit physics/Chemistry Courses Click here


