Chemistry Assignment Help with Imperfections in Solids

10.12 Imperfections in Solids
The crystal structures of ionic compounds of the type AB and AB2 discussed earlier represent ideal structures in which there is a completely ordered arrangement of constituent particles. However, in real crystals the arrangement of constituent particles is not regular and thus crystals have imperfect structures. Such imperfections are from the perfectly ordered arrangement of atoms. The disorder in a crystal of an element depends upon:
- Temperature and
- Presence of impurities
10.12.1 Defects in Stoichiometric Solids
Stoichiometric solids are those in which the numbers of positive and negative ions are exactly in the ratio indicated by their chemical formulae. The causes give rise to two types of defects as:
- Schottky defect
- Frenkel defect
Schottky defect: A pair of ‘holes’ or vacancies exists in the crystal lattice due to one cation and one anion missing from the normal lattice sites. The crystal as a whole remains neutral because the number of missing cations and anions remains the same. This sort of defect occurs in highly ionic compounds with a high coordination number and where the ions (both cations and anions) are of similar size. Alkali metal halides such as NaCl, KCl, KBr, and CsCl show this defect. In NaCl crystal, at room temperature, there will be one Schottky defect per 1016 ions.
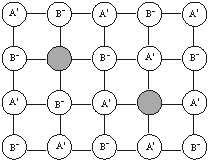
Frenkel defect: When a ‘hole’ or ‘vacancy’ exists in the crystal lattice because an ion occupies an interstitial lattice site, it gives rise to Frenkel defect. The crystal remains neutral. The defect occurs more frequently in solids which have low coordination number and possess ions (cations and anions) of different sizes. Since cations are generally smaller than anions, it is more common to find the cations occupying the interstitial sites. For example, in AgBr and ZnS crystals, Ag+ ions and Zn2+ ions are missing from their normal lattice sites and are present in the interstitial positions. AgBr, AgCl and AgI contains Schottky defects also.
10.12.2 Metal Excess Defects
These defects may arise in two ways:
Metal excess due to anionic vacancies. A negative ion may be missing from its lattice site, leaving a hole which is occupied by an electron which was associated with the negative ion and remains trapped in the vacancy thereby maintaining the electrical neutrality. When NaCl is treated with sodium vapours, a yellow coloured non-stoichiometric form of NaCl is obtained in which there is an excess of sodium ions.
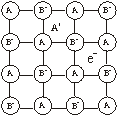
Sodium atom removes chlorine atom from the anionic lattice site leaving its electron trapped in the vacancy and makes the lattice excess of solid ions. The electrons trapped in anion vacancies are referred to as ‘F’ centres (F = Farben in German means colour).
Metal excess due to extra cations. In this case, the excess metal defect occurs when an extra positive ion occupies an interstitial position in the lattice. The free electron is trapped in another interstitial site close to the vicinity of the interstitial cation. This electron helps to maintain the electrical neutrality.
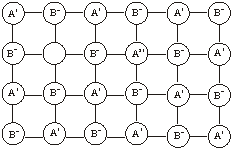
10.12.3 Metal Deficiency Defects
These defects occur in metals with variable oxidation states, i.e., the transition metals. A cation may be missing from its lattice site but the electrical neutrality is maintained when the adjacent metal ion acquires higher oxidation state Examples include FeO, FeS and NiO. Metal deficient compounds conduct electricity through positive hole conduction mechanism and are therefore p-type semi-conductors

Email Based Assignment Help in Imperfections in Solids
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Imperfections in Solids in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Imperfections in Solids. Our service is focused on: time delivery, superior quality, creativity and originality.
To submit chemistry Imperfections in Solids assignment click here


