Chemistry Assignment Help with Closest Packing in Three Dimensions

10.3 Closest Packing in Three Dimensions
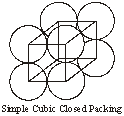
1. If in a square arrangement of layers with spheres touching each other, the second layer is placed right above the first layer of spheres then the repetition of layers become AAAA...... Such a 3-D packing gives rise to a simple cubic closed packing structure.
2. Where in the square packing of layers we have spheres not touching each other i.e.

If the second layer spheres are placed in the voids of the first layers spheres and the third layer spheres are placed in the voids of the second layer spheres then the repetition becomes AB....
Such a three dimensional arrangement leads to Body centered cubic packing.
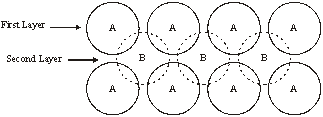
3. If in the hexagonal arrangement of layers, the second layer is placed in the voids of the first layer then we come across two types of voids
- Voids - A – Octahedral Voids
- Voids - B – Tetrahedral voids
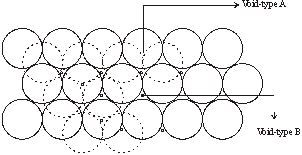
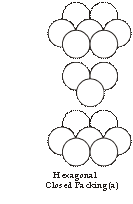
4. When the third layer is placed on the tetrahedral voids of the first layer then the repetition become ABAB because the third layer will be right above the first layer. Such a 3-D arrangement is called Hexagonal closest packing as in figure (a)
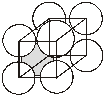
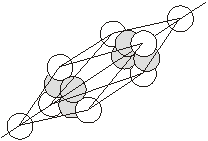
5. When the third layer is placed on the octahedral voids of the second layer then the repetition becomes ABC.... Such a 3-D arrangement is called cubic closed packing or face centred cubic closed packing.

Email Based Assignment Help in Closest Packing in Three Dimensions
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Closest Packing in Three Dimensions in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Closest Packing in Three Dimensions. Our service is focused on: time delivery, superior quality, creativity and originality.

To submit chemistry Closest Packing in Three Dimensions assignment click here.


