Chemistry Assignment Help with Cubic Unit Cell

10.7 Cubic Unit Cell
Cubic unit cell is defined as unit cell for which a = b = c and a = b = g = 90°,
Simple cubic or primitive unit cell
In primitive unit cell one atom is present at each corner of the unit cell. Atom present at corner in primitive contributes 1/8th of its total volume to the unit cell, hence effective atom(s) in unit call is/are given by ![]()
Packing fraction ![]()

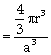
where ‘r’ is the radius of atom and ‘a’ is edge length of the unit cell, because in primitive unit cell a = 2r,
Packing fraction 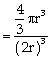
![]()
= 0.52 or 52%
This indicates that the void fraction in primitive unit cell is 48% and rest is occupied by atoms.
Body centered cubic unit cell (BCC)
In BCC one atom is present at each corner and contributes 1/8th of its volume to the unit cell and one is present at centre and contributed 100% to the unit cell, total atoms can be calculated as
Packing fraction
In given unit cell body diagonal is given by = aÖ3
or aÖ3 = 4r
or

Packing fraction 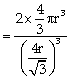 =0.68
=0.68
If means in above unit cell 68% volume is occupied by atoms and rest 38% is void.
Face centred cubic unit cell- or cubic close packing (fcc or ccp). In this type of unit cell atoms are present at all corners and faces, corner atom contributes 1/8th and face centred atom contributes ½ of its total volume to unit cell, effective atoms can be calculated as.
![]()
Packing fraction 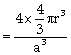
The face diagonal is given by =aÖ2
or aÖ2 = 4r
or ![]()

Packing fraction 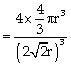
= 0.74 or 74%
Hence 26% volume of unit cell is unoccupied.
Hexagonal close packing unit cell
In hcp atoms are present at corners, top and bottom faces and inside the unit cell, corner and face atoms contribute 1/6 and ½ of its total volume respectively and three atoms situated inside the unit cell effectively contribute 100%, atoms can be calculated as
![]()
Packing friction ![]()
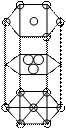
Volume of unit cell
Area of base is formed by six equilateral triangles and each equilateral triangle has side length ‘a’ (a = 2r). The area of one triangle is ![]() , hence area of the base is
, hence area of the base is
![]()
![]()
Height of the unit cell is distance between centres of top and bottom face atoms, which can be calculated as
In triangle ABC, AB = 2r
OB = r
![]()
![]()
= rÖ3
![]()
![]()
AD = 2r
![]()
![]()
![]()
or ![]()

Volume of unit cell = Area of base x height ![]()
Packing fraction 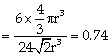
Hence in hcp and fcc. (or ccp) percent occupancy and void fraction present are same.
Email Based Assignment Help in Cubic Unit Cell
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Cubic Unit Cell in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Cubic Unit Cell. Our service is focused on: time delivery, superior quality, creativity and originality.

To submit chemistry Cubic Unit Cell assignment click here


