Chemical Kinetics - Order Of A Reaction | Graphical Representation
Methods of Determination of Order of a Reaction:
The important methods used for the following:
1. Graphical representation/method
A graphical method based on the respective rate laws can also be used.
If the plot of log (a-x) versus 't' is a straight line, the reaction follows second order.

If the plot of 1/(a-x)2 versus 't' is a straight line, the reaction follows third order.
In general, for a reaction of nth order, a graph of 1/(a-x)n-1 versus 't' must be a straight line.
For example of nth order reaction
![]() or
or ![]()
So, from this it is evident that a plot of ![]() vs (a – x)n will be straight line passing through the origin and will have its slope equal to k, the rate constant of reaction.
vs (a – x)n will be straight line passing through the origin and will have its slope equal to k, the rate constant of reaction.
This equation demands that a plot of ![]() vs log (a – x)n will be straight line of the slope equal to n, order of reaction and intercept equal to log k. For first order reaction this slope will be 1 as shown.
vs log (a – x)n will be straight line of the slope equal to n, order of reaction and intercept equal to log k. For first order reaction this slope will be 1 as shown. 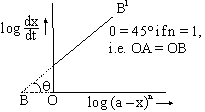
The equation may be rearranged as
log (a – x) = ![]()
Thus, a plot of log(a – x) vs. t will be straight line with slope equal to ![]() and intercept equal to log a, if the reaction is of first order.
and intercept equal to log a, if the reaction is of first order.
2. Method of integration (Hit and trial method)
The most simple method is the one in which the quantities a, x and t are determined and substituted in the kinetic equations of various orders. The equation which gives the most constant value for the specific rate constant (k) for a series of time intervals is the one corresponding to the order of reaction. If all the reactants are at the same molar concentrations, the kinetic equations are:
k = 2.303/t log10a/((a-x) ); for first order reactions.
k = 1/t [1/((a-x))-1/a]; for second order reactions.
k = 1/t [1/(a-x)2 -1/a2 ]; for third order reactions.
What is Arrhenius equation?
Answer: In Chemical reactions, it is a well-known fact that raising the temperature increases the reaction rate. An equation that represents the dependence of the rate constant k of a reaction on the absolute temperature T is well known as Arrhenius Equation.
k = A * exp (–Ea/RT)In its original form the pre-exponential factor A and the activation energy Ea are considered to be temperature-independent.
Where
k = rate constant, Ea = activation energy, R = 8.314 [ J • mol -1 • K -1 ]
T = absolute temperature in degrees Kelvin, A = pre-exponential or frequency factor
Note* [A = p • Z, where Z is the collision rate and p is a steric factor. (Z turns out to be only weakly dependant on temperature.)]
What is the unit of k for nth order reaction?
Answer: Suppose A is concentration of reactant.
Rate = > ( d[A]/dt ) = k[A]n where [A] is in mol / lit and t is in sec
(mol/lit) / sec = k (mol/lit)n
Units of k = mol1-n litn-1 sec-1
We provide Online Assignment Help services to the students in Chemical Reactions, Chemical Kinetics - Order Of A Reaction(Graphical Method). You can find answers to all of your doubts in Chemistry, Chemical Reactions and Chemical Kinetics. We at assignmenthelp.net provides homework, Assignment Help services to the School, College/University level students across the globe. Our expert online tutors are available 24/7 to help you in Chemistry. Our quality assignment service is focused on: on time delivery, originality and plagiarism free.
To submit assignments in chemistry Click here


