Chemistry Assignment Help with Arrhenius Theory
4.7 Effect of Temperature on the Reaction Rate (Arrhenius Theory)
Temperature has very marked effect on the reaction rate. It has been found that the rate of most homogeneous reaction are nearly doubled or tripled by 10° rise in temperature.
The ratio of the rate constant of a reaction at two different temperatures differing by 10° i.e. kt+10/kt is known as temperature coefficient of reaction rate. This ratio also depends upon temperature and two temperatures generally selected are 25°C and 35°C. If a reaction has a temperature coefficient of reaction rate equal to 3, then raising its temperature from 25°C to 65°C, the rate will increase by nearly 3 ´ 3 ´ 3 ´ 3 i.e. 81 times. 
In order to explain the effect of temperature on the reaction rate. Arrhenius proposed a theory of reaction rate which states as follows:
(i) A chemical reaction takes place by collision between the reactant molecules, and collision to be effective the colliding molecules must posses some certain minimum energy called threshold energy of the reaction.
(ii) Reactant molecules having energy equal or greater than the threshold are called active molecules and those having energy less than the threshold are called passive molecules.
(iii) At a given temperature there exists a dynamic equilibrium between active and passive molecules. The process of transformation from passive to active molecules being endothermic, increase of temperature increases the number of active molecules and hence the reaction.
Passive molecules ![]() Active molecules, DH = +ve
Active molecules, DH = +ve
4.7.1 Concept of energy of activation (Ea)
The extra amount of energy which the reactant molecules (having energy less than the threshold) must acquire so that their mutual collision may lead to the breaking of bond(s) and hence the reaction, is known as energy of activation of the reaction. It is denoted by the symbol Ea. Thus,
Ea = Threshold energy – Actual average energy
Ea is expressed in kcals mole–1 or kJ mole–1
The essence of Arrhenius Theory of reaction rate is that exists an energy barrier in the reaction path between reactant(s) and product(s) and for reaction to occur the reactant molecules must climb over the top of the barrier which they do by collision. The existence of energy barrier and concept of Ea can be understood from the following diagram.
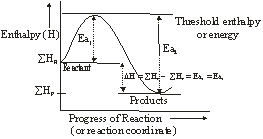
![]() = Summation of enthalpies of reactants
= Summation of enthalpies of reactants
![]() = Summation of enthalpies of products
= Summation of enthalpies of products
DH = Enthalpy change during the reaction
Ea1 = Energy of activation of the forward reaction (FR)
Ea2 = Energy of activation of the backward reaction (BR)
4.7.2 Arrhenius Equation
The variation equilibrium constant of a reaction with temperature is described by following equation of thermodynamics which is as follows
k = Ae–Ea/RT
Above equation is integrated form of Arrhenius equation. The constant A called pre-exponential factor since it is somewhat related with collision frequency. It is a constant for a given reaction. The Arrhenius equation is as follows:
![]()
With the help of this equation it is possible to calculate Ea of a reaction provided, rate constants of reaction at two different temperatures are known.

Email Based Assignment Help in Chemical Kinetics
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Arrhenius Theory in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Arrhenius Theory. Our service is focused on: time delivery, superior quality, creativity, originality and plagiarism free.
To submit chemistry Arrhenius Theory assignment click here.


