Chemistry Assignment Help with Degree of Dissociation
5.8 Degree of Dissociation
Degree of dissociation is the fraction of a mole of the reactant that underwent dissociation. It is represented by 'a'.
5.9 Relation between Vapour Density and Degree of Dissociation

The relation between vapour density and the degree of dissociation can be established only for a gaseous equilibrium who’s KP exists. For example,
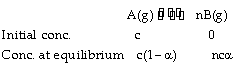
Total concentration at equilibrium = c – ca + nca = c [1 – a + na] = c [1 + a (n – 1)]
Assuming that all the gaseous components at equilibrium behave ideally, we can apply ideal gas equation.
PV = nR =\ V.D =
As pressure of the system is given by,
V.D =
where r is the density of the gas or gaseous mixture expressed in g/litre.
If the equilibrium reaction is established in a closed vessel, then vapor density will be inversely proportional to the number of moles of the gaseous species as the density of the gaseous mixture (r) is a constant.
\
Let the initial vapour density and vapour density at equilibrium be ‘D’ and ‘d’ respectively, then for the given equilibrium
or
or
\
where ‘n’ represents the number of moles of gaseous product given by 1 mole of the gaseous reactant.

Email Based Assignment Help in Chemical Equilibrium
We are the leading online Assignment Help provider. Find answers to all of your doubts regarding the Degree of Dissociation in chemistry. We at assignmenthelp.net provide homework, Assignment Help to the school, college or university level students. Our expert online tutors are available to help you in Degree of Dissociation. Our service is focused on: time delivery, superior quality, creativity and originality.
To submit chemistry Degree of Dissociation assignment click here.


