States Of Matter Assignment Help
States Of Matter
We know matter exists in three different states.
(i) Solid (ii) Liquid (iii) Gas
and these states are inter convertible into one another. A substance is solid if its melting point and boiling point above room temperature. A substance is liquid if its melting point is below room temperature and boiling point is above room temperature. A substance is gas if its melting and boiling points are less than the room temperature.
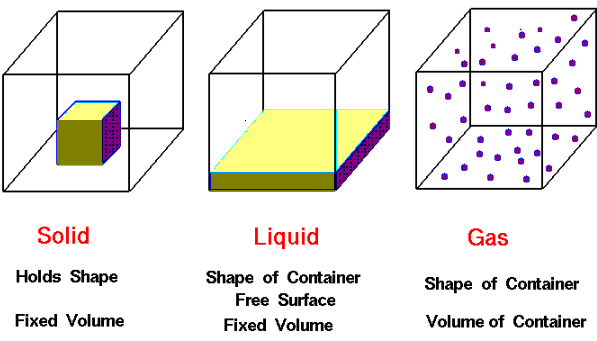

The existence of these three states of matter is due to the inter molecular force of attraction which is again dependent on temperature. With the rise in temperature the molecular energy of substances also increases and state changes.

| Solid State | Liquid State | Gaseous State |
| The intermolecular force of attraction is very high. As a result it has definite shape and volume. | Intermolecular force of attraction is less compared to that of solid as a result it has definite volume but no definite shape. | Intermolecular force of attraction is negligible as a result it has neither definite shape nor volume. |
| High density. | High density but less than solid. | Low densities. |
| Incompressible, because the molecules are packed closely to each other. | Slightly compressible. | Highly compressible because large space is there between any two molecules. |
| Molecules of solid have less energy. | Molecules of liquid have high energy. | Most energetic. |

We are offering the following main topics for assignment like:
- Gas Laws
- Dalton's Law of Partial Pressure
- Graham's Law
- Kinetic Theory of Gases
- Kinetic Energy
- Kinds of Molecular Speeds
- Real Gases
- Compressibility Factor (Z)
Homework Help For State Of Matter
assignmenthelp.net provides best Online Assignment Help service in State of Matter for all standards. Our Tutors provide their high quality and optimized Tutorial help to fulfill all kinds of needs of Students.
To submit Work Power And Energy assignment click here


