Chemistry Homework Help With Graham's Law
Graham's Law
Diffusion : Movement of particles from higher to lower concentration is called diffusion. Gases occupy all the available space due to this property.
Effusion : The movement of particles through small orifice is called effusion. It take place due to difference in pressure of inner and outer gases.
Rate of diffusion or effusion : It may be represented as volume occupied by the gas per unit time or decrease in pressure of gas per unit time or moles of gas diffused or effused per unit time.
Graham's Law : The rates of diffusion or effusion of gases under identical conditions is inversely proportional to square root of their vapour density. Mathematically,
![]()
 This law is used in comparing the rates of diffusion or effusion of gases, under similar conditions.
This law is used in comparing the rates of diffusion or effusion of gases, under similar conditions.
![]()
This law gives better results at low pressure.
This law is obeyed by ideal as well as non-ideal gases.
If the gases are at different pressure, the law may be modified as
![]()
The rate of effusion of gases is directly proportional to the surface area of orifice.
If a gaseous mixture of n1: n2 mole ratio is to be separated by diffusion method, then after n times of diffusion process, the mole ratio becomes
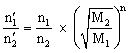
Comparison of times taken for the same volume of two gases
![]()
Homework Help For Graham's Law
assignmenthelp.net provides best Online Assignment Help service in Graham's Law for all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Graham's Law assignment click here.


