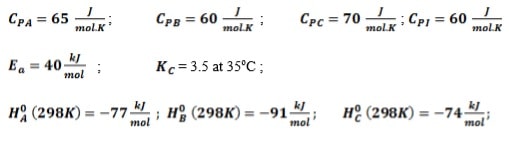Reactor Technology IV Assignment
{`VAAL UNIVERSITY OF TECHNOLOGY
DEPARTMENT OF CHEMICAL ENGINEERING
SUBJECT: REACTOR TECHNOLOGY IV
SUBJECT CODE: EHTRN4A
`}
Question 1 Marks 20
Ethane is pyrolysed in a plug flow reactor at 815°C and 2.87 atm:
C2H6 ⇔ C2H4 + H2
At the reaction conditions equilibrium is represented by Kp = PPBPAC =1.45atm
Feed to the reactor is pure ethane. Calculate the molar feed rate of ethane that would be required to achieve 90% of the equilibrium conversion in a 100 dm3 reactor. k = 1.1 min-1 at 815°C.
Question 2 Marks 20
The following reaction is taking place in a PBR: A + B {`->`} 2C
The feed consists of 15% inerts with the remainder being a stoichiometric mixture of the 2 reactants. The feed enters the reactor at the rate of 1.5 kmol/h at 260 oC and 2 atm, the pressure drop parameter 𝛼?=0.004 𝑘𝑔−1. The reaction rate and the rate constant are given by:
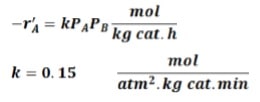
Calculate the catalyst weight required to achieve 75% conversion for the following cases:
- In the absence of pressure drop
In the presence of pressure drop
Question 3 Marks 20
The elementary, liquid-phase, irreversible reaction A+B →C is is to be carried out in a flow reactor. Two reactors are available, an 800 dm3 PFR that can only be operated at 300 K and a 200 dm3 CSTR that can be operated at 350 K. The two feed streams to the reactor mix to form a single feed stream that is equal molar in A and B, with a total volumetric flowrate of 10 dm3/min. Which of the two reactors will give us the highest conversion?
Additional information:
- At 300 K, k = 0.07 dm3/mol-min
- E = 85000 J/mol-K
- CA0 before mixing = CB0 before mixing = 2 mol/dm3
- vA0 = vB0 = 0.5*v0 = 5 dm3/min
Question 4 Marks 20
A reactor bomb fitted to a sensitive pressure measuring device is filled with a gas containing 40% A and 60 % inerts to a total pressure of 4 atm at 25oC. The temperature of the bomb was rapidly raised to 100oC. At this temperature, A decomposes according to the stoichiometry:
A → 2B
|
Time (min) |
1 |
2 |
4 |
6 |
8 |
10 |
12 |
|
Total pressure in reactor at 100oC |
5.19 |
5.36 |
5.66 |
5.9 |
6.1 |
6.26 |
6.4 |
Assume there is no significant reaction at 25oC and during the rise to 100oC, determine the rate law of decomposition of A.
Question 5 Marks 15
Tests were run on a small experimental reactor used for decomposing nitrogen oxides in an automobile exhaust stream. In one series of tests, a nitrogen stream containing various concentrations of NO2 was fed to a reactor and the kinetic data obtained is shown in figure 1 below. Each point represents one complete run. The reactor operates essentially as an isothermal backmix reactor (CSTR).
- What can you deduce about the apparent order of the reaction over the temperature range studied?
- What is the specific rate of reaction for the test conducted at 300oC.
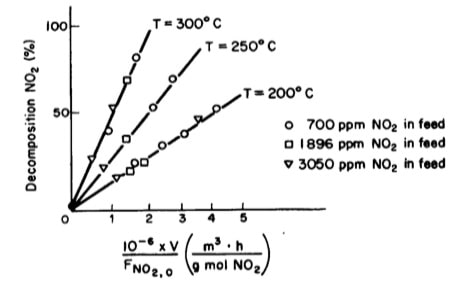
xFigure1: Plot of percent decomposition of NO2 V/FAO
Question 6 Marks 25
The following elementary reversible gas phase reaction is carried out adiabatically in a PFR. 2A ⇔ B + C
A feed containing 75% A and 25 % inert enters the reactor at the rate of 120 kmol/h, temperature of 310K and pressure of 10 atm. The specific reaction rate at 325K is 0.5 dm3.mol-1.s-1. Assuming the pressure drop is negligible:
- Determine the expression for equilibrium conversion
- By making use of Simpson’s three-eights rule, determine the volume necessary to achieve 72% conversion of A
- Determine the maximum conversion that can be achieved for this exothermic reaction as well as the adiabatic temperature
Additional information: