AES1300 Properties of Hydrocarbons and Oilfield Fluids
1. Phase diagram for pure component:
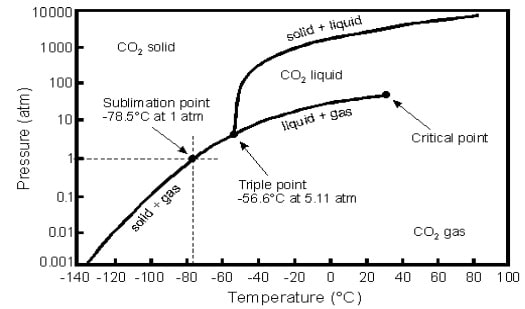
Fig.1 Pressure-Temperature diagram for CO2
- We perform an experiment on CO2 in a closed container (V = const) with the following steps:
- The experiment starts at T = 20oC and p = 200 bars
- We drop the pressure in container down to p = 10 bar until T equals -60 o
- Next, keeping the same T, we increase p up to 200 bars again
- We heat container up to T = 20oC again
Describe all states of CO2 at each stage of the experiment. Describe also the assumptions you made in order to choose a particular connection path. Use the diagram and report (approximate) changes in p and T connected with the state of CO2 during each stage of experiment. Are we going to arrive to the same state by the end of the experiment? Explain why.
- Recall the equation of Clausius and the Clausius-Clapeyron describing the vapor pressure curve. Give the Clausius equation before integration as dp/dT and the Clausius-Clapeyron equation after the integration a p = f(T). Which assumptions are made to allow the integration?
- The molar enthalpy of vaporization can be described by the so-called Trouton’s law. For a certain component Trouton’s law can be written as:
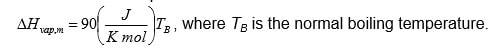
Determine the molar enthalpy of vaporization in J/mol if the component boils at 1200C at a pressure of 1 bar.
- Give the equation to describe the vapor pressure curve for this component (based on the Clausius-Clapeyron equation). Plot the vapor pressure in bars as function of the temperature in the range from 10 to 150o Use MATLAB.
- Determine for this component the boiling point (temperature) at 50 bars (assuming that this pressure is lower than the critical pressure).
- Deliver short report of the MATLAB task in Section 1
- A hydrocarbon mixture having the composition given in table 1 is submitted to a petroleum laboratory for analysis. The mixture is maintained at a pressure of 1 atmosphere and a temperature of 60 0
- Write down all possible structural formulae of the compounds. Explain why you choose particular structure.
- Which components are isomers?
- Would you expect the hydrocarbon mixture to be a liquid or a gas phase? Explain.
- How much water would be produced upon burning 250 g of the hydrocarbon mixture in the presence of oxygen?
Table 1
|
Hydrocarbon |
Weight % Boiling |
Freezing point At 1 atm [K] |
Molecular weight [g mol-1] | |
|
temperature at 1 atm [K] | ||||
|
Methane |
25.7 |
111.66 |
90.69 |
16.043 |
|
Ethane |
6.4 |
184.55 |
90.35 |
30.07 |
|
Propane |
5.3 |
231.02 |
85.47 |
44.097 |
|
Isobutane |
0.2 |
261.34 |
113.54 |
58.123 |
|
n-Butane |
2.4 |
272.66 |
134.79 |
58.123 |
|
Benzene |
9.8 |
353.24 |
278.68 |
78.114 |
|
Toluene |
8.5 |
383.79 |
178.16 |
92.141 |
|
Ethylbenzene |
9.2 |
409.36 |
178.18 |
106.167 |
|
o-Xylenes |
11.3 |
417.59 |
247.97 |
106.167 |
|
1,2,4-Trimethylbenzene |
9.9 |
442.49 |
229.35 |
120.194 |
|
CO2 |
11.3 |
-. |
216.58 |
44.01 |
- Upon lowering the temperature of 500 g of the above mentioned hydrocarbon mixture below 7 0C we obtain a liquid residue of the aromatic compounds while the non-aromatic hydrocarbons remain essentially in the gaseous phase.
- Determine the composition of the gas obtained and then the composition of the liquid residue. Compositions are required in weight percent (wt%).
- How much carbon dioxide would be produced by combustion of the gas from exercise a?
- In 4-component system representing typical reservoir fluid, at most 3 phases can co-exist simultaneously (aqueous and liquid and vapour hydrocarbon). Describe the dimensions (point, line, area, volume) of all possible combination of phases.


