Prodrugs Sample Assignment
ABSTRACT
The prodrug design concept has been widely used nowadays in order to alter the undesirable properties of active drugs. These undesirable properties of the active drugs which can be solved by the prodrugs include chemical instability, reduced aqueous solubility, poor lipophilicity, rapid excretion from body, poor distribution across the biological membranes and shorter half life. Prodrugs can be used with different functional groups which help to overcome these undesirable properties. They help in improving the drug targeting, thus large amount of drug is available at the target site to produce the desirable pharmacological action. This assignment highlights the new classification of prodrugs, their role in drug design and the most commonly used functional groups on parent drugs. It provides an insight into the
modern mechanisms of prodrug design.
INTRODUCTION
Prodrugs are the inactive, bioreversible form of the active drug molecule. Upon enzymatic or chemical degradation, they release the active drug molecule at the targeted sites which then produces the desirable pharmacological response. (Huttunen 750) In the phases of drug discovery and its design, prodrugs have a very important role to improve the biochemical, pharmacokinetic, biopharmaceutical and pharmacodynamic properties of the active drugs. A total of 5-7% of the total drugs approved are prodrugs. Implementation of prodrug design in the early phase of drug discovery is very much beneficial. (Rautio 255)
Prodrugs are beneficial in improving and reducing water solubility (as desired), enhancing lipid solubility, improving drug stability, reducing the toxic effects of the active drug and increasing the duration of action of the drugs. For some drugs which are superior in terms of efficiency, a shorter half life leads to frequent administration of the drug and thud noncompliance issues. In such situations, methods focusing on sustained release mechanisms should be used. This can be achieved by formulating the drug as a prodrug.
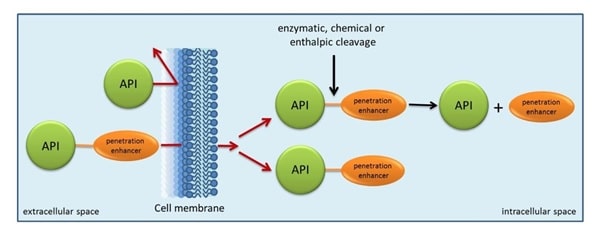
Fig 1: Concept of prodrugs
Source: http://www.ionovation.com/index.php/ionochem
CLASSIFICATION OF PRODRUGS
There are many methods of classifying prodrugs. Classifying them on the basis of their cellular sites of conversion into the final active form of the drug is more appropriate. There are some concerns regarding the prodrug development such as whether the prodrug completely converts into active form and whether it contributes to the toxicity profile of the active drug. Thus, in order to assess the safety and efficacy of prodrugs, a classification system based on the cellular sites of conversion will be more appropriate as it provides the kinetics of the conversion procedure. (Wu 78)
Fig 1: Classification of prodrugs
|
Prodrug type |
Cellular site of conversion |
Subtypes |
Tissue location of conversion |
Examples |
|
Type I |
Intracellular |
A |
Therapeutic Target Tissues/Cells |
• Acyclovir • 5-Flurouracil • Cyclophosphamide • Diethlstilbestrol diphosphate • L-Dopa • 6-Mercaptopurine • Mitomycine C • Zidovudine |
|
B |
Metabolic Tissues (liver, GI mucosal cell, lung, etc.) |
• Cabamazepine • Captopril • Carisoprodol • Heroin • Phenacetin • Primidone • Suldinac | ||
|
Type II |
Extracellular |
A |
GI fluids |
• Lisdexamfetamine • Loperamide oxide • Oxyphenisatin • Sulfasalazine |
|
B |
Systemic Circulation and Other Extracellular Fluid Compartments |
• Acetylsalicylate • Chloramphenicol succinate • Dihydropyridine pralixoxime • Fosphenytoin | ||
|
C |
Therapeutic Target Tissues/Cells |
• ADEPs • GDEPs • VDEPs |
COMMON FUNCTIONAL GROUPS USED IN PRODRUG DESIGN
About 50% of the prodrugs which are available in the market are ester prodrugs. These are activated through enzymatic hydrolysis by esterases. The main function of these ester prodrugs is to enhance the lipophilicity and to improve aqueous solubility. (Zawilska 3)
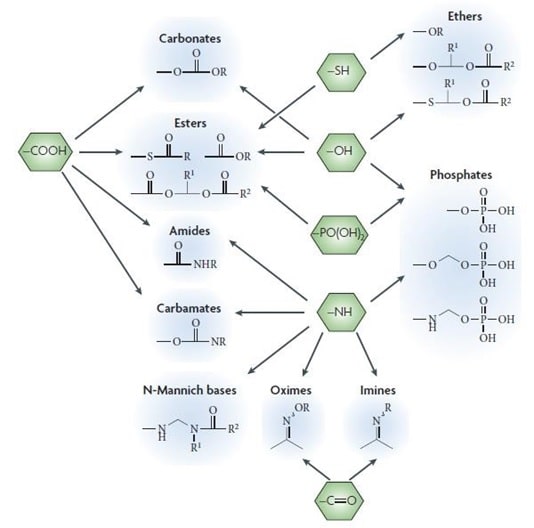
Fig 2: Common functional groups on the drug applied in prodrug design Source: (Zawilska 3)
ROLE OF PRODRUGS IN DRUG DESIGN
Prodrugs approach can be used at different stages of drug development. The important stages at which prodrug design is necessary to overcome the undesirable properties of the drug are pharmaceutical phase and pharmacokinetic phase.
1. Pharmaceutical phase
The undesirable properties of drugs arise due to
- Aesthetic properties -Odour, taste, pain upon injection
- Formulation properties -solubility, stability, polarity
Prodrug design to overcome pharmaceutical undesirable properties
a) Masking taste and odour
The undesirable taste is due to interaction of the drug with the taste receptors and high aqueous solubility. Hence, decreasing the solubility of the drug solves the problem. Claasic example of this approach is Chloramphenicol, which is bitter in taste. To mask its taste its solubility is reduced by converting it to a less soluble ester, Chloramphenicol palmitate. It is tasteless and undergoes hydrolysis by pancreatic lipase into chloramphenicol invivo. (Ambrose 223)
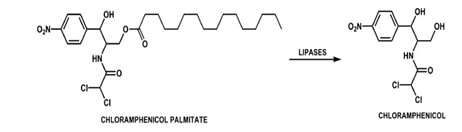
Fig 3: Enzymatic conversion of prodrug Chloramphenicol palmitate to chloramphenicol
Source: Parise Filho, Roberto, Polli, Michelle Carneiro, Barberato Filho, Silvio, Garcia, Monique, & Ferreira,
Elizabeth Igne. “Prodrugs available on the Brazilian pharmaceutical market and their corresponding bioactivation pathways”. Brazilian Journal of Pharmaceutical Sciences. 2010; 46(3):393-420.
b) Reducing the pain at the site of injection
Pain at the site of injection is caused due to poor water solubility and acidic nature. Phenytoin, an antiepileptic drug when given as intramuscular injection causes pain. This can be overcome by formulating Phenytoin as Fosphenytoin, a phosphate ester with high aqueous solubility and pH at 12. (Boucher 779)
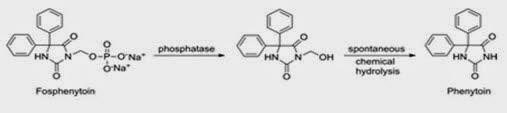
Fig 4: Enzymatic conversion of Fosphenytoin to phenytoin (Source: Boucher 1996)
c) Solubility alteration
The alteration of solubility can be done by the prodrug design. In some instances where enhanced solubility is desirable, for example parenteral administration of chloramphenicol succinate with enhanced solubility is the result of prodrug approach. (Ambrose 224)
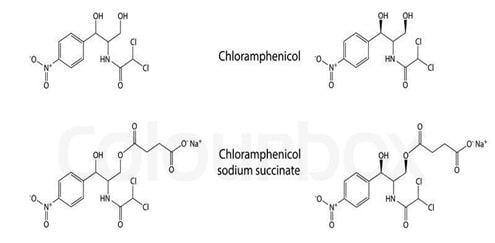
Fig 5: Prodrug of Chloramphenicol (Source: Ambrose 224)
d) Enhancing the chemical stability of drug
The chemical stability of the drug is required for its pharmacological effect at the target tissues for a sustained duration. Chemically unstable drugs lose their efficacy due their stability issues. Hence prodrug approach is helpful in such situations.
Ampicillin, a beta lactam antibiotic undergoes aminolysis due to its NH2 group on side chain, which leads to chemical instability. Hetacillin, a prodrug of ampicillin forms a ring to lock the offending nitrogen. Upon administration, hetacillin decomposes to ampicillin and acetone. (Patrick 264)
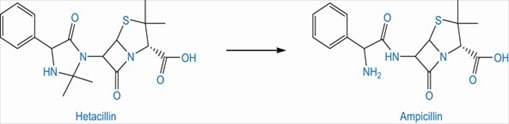
Fig 6: Conversion of hetacillin to active form, ampicillin (Source: Patrick 264)
2)Pharmacokinetic phase
Pharmacokinetics of the drug involves four stages namely absorption, distribution, metabolism and excretion. Thus the problems arise at any of these stages to make the drug with undesirable properties. These properties include
- lack of complete absorption of the drug
- metabolism of drug in the pre systemic phase leading to improper systemic delivery of drug
- lack of site specificity
- toxicity problem
a)Enhancement of absorption- oral, ophthalmic and percutaneous
Some of the highly polar therapeutic drugs such as water soluble vitamins, purine and pyrimidine derivatives, antibiotics, phenytoin have the barrier to gastrointestinal absorption. This can be solved using prodrug approach. The prodrug of dopamine, L-Dopa is has high aqueous solubility but it is actively transported through the blood brain barrier by specific L- amino acid transporter. It releases dopamine in the CNS by decarboxylation process. (Patrick 265)
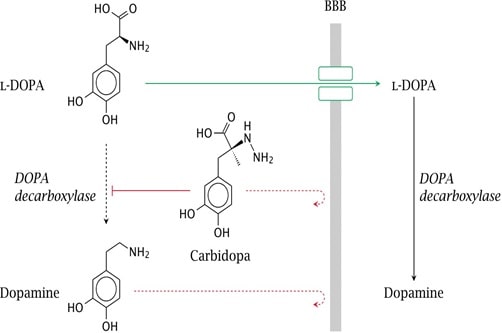
Fig 7: Conversion of L-Dopa to Dopamine at blood brain barrier (Source: Patick 265)
The therapeutic benefits of a highly polar drug, epinephrine in the treatment of glaucoma is limited because of its greater aqueous solubility. To enhance the effectiveness of epinephrine in the treatment of glaucoma, the acylation of phenolic hydroxyl groups of epinephrine is done to result in a prodrug, dipivalyl derivative of epinephrine. The lipid solubility of this prodrug allows it to cross the lipoidal barrier of cornea. (Hussain 1510)
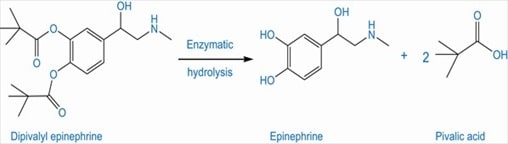
Fig 8: Epinephrine prodrug (Source: Hussain 1510)
- Reducing the pre systemic metabolism
The drugs are rapidly degraded in acidic nature of stomach or due to the enzymes present in the gastric mucosa. This rapid degradation is known as first-pass metabolism. This can be prevented by masking the functional groups responsible for it. This concept can be applied to the corticosteroidal drugs by which the absorption and bioavailability of the parent steroids can be enhanced. (Haavik 1080)
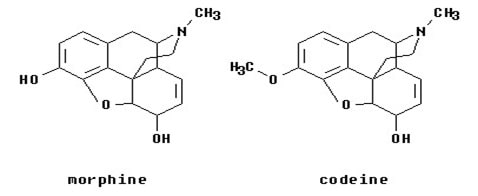
Fig 9: Prodrug of morphine (Source: Haavik 1080)
- To prolong the duration of action
Some drugs have shorter half life due to which they require frequent docing. This may lead to non-compliance of the patient to the drug. Thus in such cases, prodrug approach can be applied to prolong the duration of action of the drug.
This can be applied to the neuroleptic drugs in order to maintain the plasma concentrations steadily. For fluphenazine, hepatanoate and decanoate esters can be administere with sesame oil for sustained release.
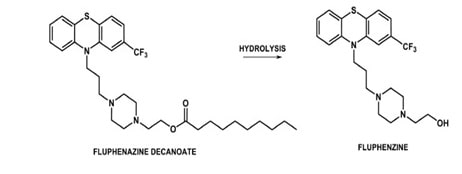
Fig 10: Fluphenazine prodrug (Source: Brazilian Journal of Pharmaceutical Sciences)
- To reduce the systemic toxicity of drugs
The drugs should have desirable pharmacologic properties at the target site and the toxicity profile should be low. In order to achieve this, the prodrug approach should be followed for certain drugs.
Several NSAIDs exhibit the property of gastric irritation due to damage of the gastro intestinal mucosa by free carboxyl groups. Even sulindac has the same problem of toxic side effects.
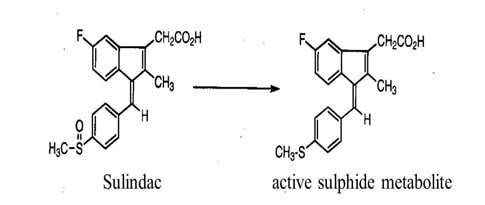
Fig 11: Sulindac prodrug (Source: Brazilian Journal of Pharmaceutical Sciences)
RECENT ADVANCES IN PRODRUG DESIGN
Molecular revolution has introduced new concepts of prodrug design such as attaching the promoieties to the molecule of interest, and thereby targeting the transporters and enzymes.
Targeting transporters
The treatment of influenza is done by two drugs zanamivir and oseltamivir. There are several evidences indicating the resistance of oseltamivir in literatute, even though it is a prodrug. The prodrug concept was applied to zanamivir, by improving theoral absorption. This can be done by targeting PEPT1 for transport mediated absorption. (Dahan 16492)
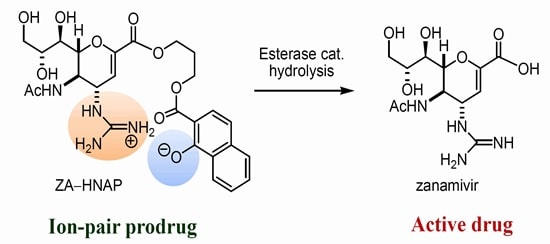
Fig 12: Zanamivir prodrug (Source: Dahan 16492)
Targeting enzymes
The prodrugs when administered into the body get activated by the degradation which may be chemical or enzymatic. The enzymes commonly involved in the degradation of prodrugs are acetylcholinesterase, cholinesterase and carboxylesterase. This approach can be successfully applied in the cancer chemotherapy. (Dahan 16495)
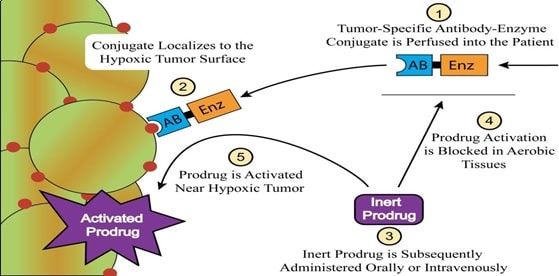
Fig 12: Prodrugs in cancer chemotherapy (Source: Dahan 16495)
CONCLUSION
The molecular revolution has altered completely the prodrug approach. The old approach of prodrugs was aimed at altering the physicochemical, pharmaceutical and pharmacokinetic properties. The novel methods of prodrugs targeting the enzymes and transporters have been introduced. This approach has taken its stand from the beginning of the drug development phase. Applying the prodrug approach may significantly lower the costs and result in predictable outcomes. Further large scale studies need to be conducted in this area to grab the maximum benefits.
WORKS CITED
Ambrose, P. J. 'Clinical Pharmacokinetics of Chloramphenicol and Chloramphenicol
Succinate.' Clin Pharmacokinet. 1984; 9(3): 222-38
Boucher, B. A. 'Fosphenytoin: A Novel Phenytoin Prodrug.' Pharmacotherapy. 1996; 16(5): 777-91.
Dahan, A., Zimmermann, M. &Ben-Shabat, S. “Modern prodrug design for targeted oral drug delivery”. Molecule . 2014; 19: 16489-16505.
Haavik, P. E. “Codeine Is a Prodrug--the Active Agent Is Morphine” . Tidsskr Nor
Laegeforen. 2000 Mar 30;120(9):1080
Hussain, A., and J. E. Truelove. 'Prodrug Approaches to Enhancement of Physicochemical Properties of Drugs Iv: Novel Epinephrine Prodrug.' J Pharm Sci . 1976; 65(10):
1510-2.
Huttunen, M., Raunio, H. &Rautio, J. “Prodrugs- from Serendipity to Rational Design”.
Pharmacological reviews. 2011; 63(3): 750-771.
Patrick, G. L. An introduction to Medicinal Chemistry, 5thedn. Oxford: Oxford University
Press. 2013.
Rautio, J., Kumpulainen, H., Heimbach T., Oliyai R., Oh D., Jarvinen T. & Savolainen J.
'Prodrugs: Design and Clinical Applications.' Nat Rev Drug Discov. 2008; 7(3):
255-70.
Wu K.M. “A new classification of prodrugs: Regulatory perspective”. Pharmaceuticals.
2009; 2: 77-81.
Zawilska, J., Wojcieszak, J. &Olejniczak, B. “Prodrugs: A challenge for the drug development”. Pharmacological Reports. 2013; 65: 1-14.


