Literature review on antimicrobial resistance
Abstract
The substantial efforts in research process have aims to incorporate various moieties functionalities in entire design of nano particle. As a collective body, the transporters can perform as a super system that can confer tolerance to a huge range of harmful compounds. In gram negative bacteria, some transporters can serve as the components of energy transducing. There are various types of multidrug transporters which have been discussed in this assignment. Along with these the different channels of membrane with their functions and also structures has have been resented in this assignment. In order to evaluate the role of RND transporters, different kinds of drug resistance mechanisms are also introduced in this literature review. The basic aim is to identify the significance and importance of antimicrobial resistance in respect to biological aspects. Other than these by addressing the barriers, features of innovative design is incorporated. Moreover it can be said that it will able to create new generation of nanotheraputics in drug delivery based on nanaoparticles.
Introduction
Biological obstacles towards drug transport are able to prevent accumulation of nanotherapeutics at diseased sites, and can limit efficacious responses in process of disease from cancer to inflammation. It can be stated that inadequate accumulation regarding therapeutics, nonspecific distribution may set barriers to drug developers. Although there are no inhibitors that are clinically available regarding exporters of bacterial multidrug, efforts in order to enhance inhibitors which are based on structural information are underway. Moreover, it can be stated that use of combination of genetic, biochemical and approaches of biophysics are able to help in reconstruction of event sequences. Antimicrobial resistance can be considered as a part of threatening the preventions of bacterial or fungal infections in the modern era. As per the report of World Health Organisation, 4,900,00 people across the globe has developed multi-drug resistant TB (World Health Organisation, 2018). This review has focused on mechanism of reactions of coupling transports which are present in outer cell membranes of Gram- negative bacteria that primarily acts as fostering factors in drug-resistance. This report addresses some specific objectives that are highlighted below:
- To evaluate the role of multidrug transporters in drug resistance
- To evaluate in detail the function and structures of RND transporters membrane channels such as MtrE, OprM and TolC
- To discuss other different mechanisms regarding drug resistance
- To discuss about the role of outer-membrane proteins in drug efflux.
Functions of tripartite multidrug transporters in drug resistance
The functions of tripartite transporters of multi drug are considered as an important one in drug resistance. As per the views of Blanco et al. (2015, p.1), the transporters are present in all living systems. Moreover, the transporters are particularly diverse and abundant in bacteria and are able to comprise 2-7% of entire protein content of bacteria. Such multidrug transporters are detected on the basis of similarity of sequences along with transporters, who are experimentally confirmed. Similarly, based on the views of Zgurskaya et al. (2015, p.100), it can be stated that phylogenetic analysis and functional studies can demonstrate that MDR transporters have been organized into various families of distinct proteins. These wide range of proteins differ in structure, transport mechanism and bioenergetics. According to Yamaguchi et al. (2015, p.327), most of transporters are found in three diverse and large superfamilies such as ABC, RND and MF that undergoes the Tat System of drug efflux.
Additionally, some of the transporters are able to organize a core of superfamilies which are smaller. It can be observed that recently a new family is engaged in cyclohexidine efflux. As per the views of Zgurskaya et al. (2015, p.320), ABC transporters are the types of transporters which are primarily active and allows substrate translocation along with binding and ATP hydrolysis. Multidrug transporters in other super families are considered as secondary transporters that use gradients of ions which are electrochemical in order to transport their diversified substrates. According to Yamaguchi et al. (2015, p.309), it can be also depicted that in bacteria both the secondary and primary transporters are ubiquitous.
However, it can be observed the relative presence of both transporters seems to maintain relation with generation of energy. Fermentative bacteria are able to depend more on primary transporters at the time when genomes of aerobic bacteria have something more than primary transporters. For example, Du et al. (2015, p.77) reported that MsbA from Salmonella typhimurium is found to provide a crystal structure that is nucleotide bound. Similarly, multidrug resistance of P-glycoprotein ABCB1 was obtained from Caenorhabditis elegans that provides a similar crystal structure that uses “open conformation” cycle for ABC exporter.
Mechanism of the transport of drug involves the process of proton translocation, supporting a proton pathway that involves the residues. Du et al. (2015, p.76) stated that the structural repeats that are present in the portion of transmembrane, usually undergo a rotation at the time of transport cycle. Stabilisation is caused as a result of substrate binding consequently triggering the movement of membrane helices and forms a water molecule chain. This results in protonation of residues, disrupting the water chain and returning of protons to periplasm. Intermonomeric communication involves the coupling in three specific states that finally leads to drug efflux. On the contrary, Weatherspoon-Griffin et al. (2014, p.2) commented that E. coli resistance via tripartite multidrug to the protamine needs to represent CpxR-mediated mechanism that functions in a completely different process when compared to the Tat System.
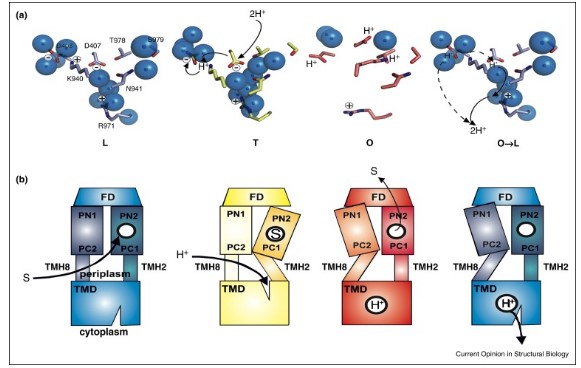
Figure 1: Proton coupling mechanism of RND transporter from E. coli.
(Source: Du et al. 2015, p.76)
Role of RND multidrug transporters in drug resistance
Exporters of bacterial multidrug are considered as transporters of intrinsic membrane which is able to act as mechanism of cellular self defence. The most important characteristics of these exporters is about the process of exporting a wide range of toxic compounds and drugs. Initially, the review section has discussed about multi drug exporters of RND type specially the major exporter in Gram-negative bacteria, AcrAB-TolC. This kind of drug exporters is tripartite complexes that comprise a transporter of cell membrane. On a similar note, Fitzpatrick et al. (2017, p.6) stated that RND exporters have specificity regarding broad substrate among other bacterial exporters of multidrug. In various cases, high rate of multi drug resistance in pathogens can be caused due to the synergetic effect of transporters and other factors of drug resistance. Transporters of Small resistance have an important role in drug resistance to the lipophilic drug. However, based on a controversy, it can be stated that MexAB has been identified as a factor of drug resistance in P. aeruginosa which reflects the expression of intrinsic pumps of efflux.
Structure and function of the outer membrane channels of RND transporters
The outer membrane of bacteria, Gram-negative is a lipid bilayer that is able to slow down drug diffusion and it also slows down biels salt into the cell. As per the views of Daury et al. (2016, p.4), it can be stated that though the internal leaflet of outer membrane is composed with glycerophospholipids, the basic components of the membrane is composed with lipopolysaccharides. This can be considered as responsible for characteristics of low permeability. Among all the families of outer membrane channel, protein belongs to factor of outer membranes. At the time of transport, OMFs are believed in transition between two states that are closed and open. Fitzpatrick et al. (2017, p.4) revealed the fact that the first OMF characterised and purified was TolC which is homotrimeric from E. coli. The author also stated that this can function with different pumps of drug efflux of RND. TolC is a stand barrel of 12 β that contains 6 loops and is a 30-Å wide. Moreover, these loops are exposed to surface of the cell. The β barrel is able to extend into periplasm as α barrel this is formed by 12 α helices. Among these six continuous and six discontinuous which can share interactions that are inter helical and similar sequences. The domain of α barrel is belted by an α or β structure which is able to comprise the equatorial domain.
TolC homologs have been crystalized since earlier,VceC, MtrE, CusC, and ,OprM, Conserve closely with the architecture of α and β barrel. According to Zgurskaya et al. (2015, p.3), the equatorial domain can be retained along with variation which is significant,especially in C terminus. Mutations in the C and N terminus can attenuate the function of TolC by highlighting significant roles of equatorial domain. The crystal structure of MtrE is able to show that this membrane channel is open to all way from the surface of the membrane and down to the domain of α helical periplasmic. In accordance to the above views, Fitzpatrick et al. (2017, p.6) has commented that the widest section of this membrane channel is situated at external surface of membrane along with the internal diameter about ∼22 Å. However, it can be stated that MtrE can be considered as high dynamic channel which is transitioning spontaneously between the closed and open states. Based on the views of Zgurskaya et al. (2015, p.2), same as TolC, this channel also have an aspirate ring at the entrance of its periplasmic. All the promoters of MtrE can able to contribute Asp405 and Asp402 in order to make two circles which are concentric regarding negative changes within internal cavity of MtrE that could have contribution to channel’s selectivity.
The most important and relevant examples regarding tripartite systems of RND are MexAB-OprM of Pseudomonas aeruginosa. X- ray structures of high resolution are easily available for OprM’s single component. This OprM possesses an organization which is tremeric and also is consisting in a transmembrance domain that is 4 nm long. In respect to the above fact, Daury et al. (2016, p.3) has opined that OprM can be sandwiched between two various lipid membrane which can reveal intermembrane distance near about 21-nm. It can be reported that reconstitution regarding native MexAB–OprM as well as AcrAB–TolC can be complex for using nanodisc technology. The reconstitution of OprM was achieved with contrast with two lipids which are reported to make ND of 10 nm sized diameter.
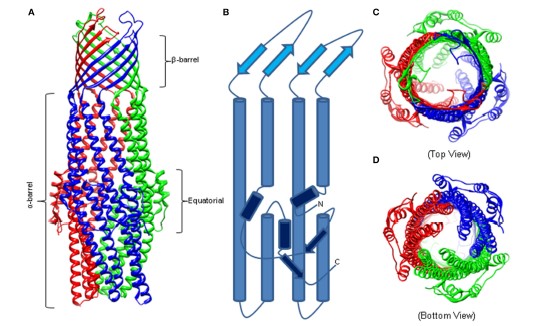
Figure 2: Structure of HomotrimericTolC
(Source: Zgurskaya et al. 2015, p.3)
Explanation of other mechanisms of drug resistance
Infections regarding multidrug resistant bacteria are increasing across the globe in a significant manner. Thus it has become essential to comprehend the underlying mechanisms of the same for discovering new therapeutics. Blair et al. (2015, p.1) highlighted that efflux pumps of Gram-ve bacteria is substituted by the G288D substitution in the AcrB. This is identified as the transporter of resistance-nodulation division in the efflux pump system of AcrABTolC tripartite MDR. Antibiotic resistance is considered as multifactorial and multifaceted. Moreover according to Alibert et al. (2017, p.2), multidrug resistance is ascribed to active presence of various mechanisms of resistance. The MDR phenotype has been enhanced to both intrinsic and acquired mechanisms. The additional resistance mechanism includes mutation in target of antibiotic. On the contrary, Melnyk et al (2015, p.274) has stated that there are another mechanism which is peristaltic mechanism which is able to engage substrate interactions and which has a relation to MDR efflux pumps in antimicrobial resistance. As per the views of Melnyk et al. (2015, p.273) prokaryotic microbes is able to gain resistance called de novo by the evaluation of transfer regarding resistance. The basic topics which has been discussed in respect to mechanism can release these nanoparticles (Durán et al. 2016, p.3).Moreover, in this context, it can be identified from the views of Ling et al. (2015, p.455), that Teixobactin has an important role against drug resistance and also pathogen.
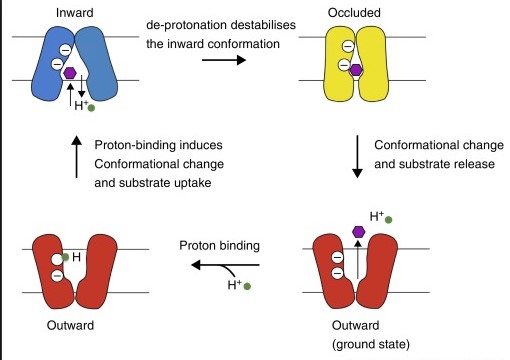
Figure 3: Diagram showing mechanism of RND transporters
(Source: Du et al. 2015, p.77)
Conclusion
Multidrug resistance of cancer cell and pathogens are considered as a serious problem of current chemotherapy. The resistance of multi drug can reflect the various factors of drug resistance such as detoxifying antibiotics of enzymes, permeability barriers and mutations regarding drug target. The molecular mechanisms related to recognition of multi drugs and export by the transporters of RND type has been exposed through the determinations of crystal structure over last decades. Along with the literature review regarding the antimicrobial resistance, it can be identified that the recognition of multidrug is based on process of binding multisite drug within binding pockets which are voluminous. The multiple entrances are able to allow the export of both hydrophilic and hydrophobic compounds. Functional rotation mechanism can be considered as effective in process of mediation of drug efflux. In this review, the important role or functions of tripartite multidrug transporters has been clearly discussed along with the structure of outer membrane channels such as MtrE, TolC,and OprM. Besides these, the other mechanisms related to the drug resistance have been exposed in detail in order to meet the aims of this assignment.
Most importantly, future studies can be able to address questions which are concerned about the difficulties in identification of substrates which are bound within asymmetric structures. It can be depicted that the process of addressing these in an exciting manner will improve studies related to protein dynamics and also determinations regarding tripartite complex. Moreover, the impact of significance of antimicrobial resistance can be evaluated from various perspectives which include societal perspective. The present days understanding regarding the importance of antimicrobial resistance upon the society can be considered as limited. Through this project activity it can be detected that it costs much to do experiments with antibiotic resistance. It can be concluded that the future study regarding the significance of resistance will be effective in process of decision making.
Reference list
Alibert, S., N’gompaza Diarra, J., Hernandez, J., Stutzmann, A., Fouad, M., Boyer, G. and Pagès, J.M., (2017). Multidrug efflux pumps and their role in antibiotic and antiseptic resistance: a pharmacodynamic perspective. Expert opinion on drug metabolism & toxicology, 13(3), pp.301-309.
Blair, J.M., Bavro, V.N., Ricci, V., Modi, N., Cacciotto, P., Kleinekathӧfer, U., Ruggerone, P., Vargiu, A.V., Baylay, A.J., Smith, H.E. and Brandon, Y., (2015). AcrB drug-binding pocket substitution confers clinically relevant resistance and altered substrate specificity. Proceedings of the National Academy of Sciences, p.201419939.
Blair, J.M., Webber, M.A., Baylay, A.J., Ogbolu, D.O. and Piddock, L.J., (2015). Molecular mechanisms of antibiotic resistance. Nature Reviews Microbiology, 13(1), p.42.
Blanco, E., Shen, H. and Ferrari, M., (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature biotechnology, 33(9), p.941.
Daury, L., Orange, F., Taveau, J.C., Verchère, A., Monlezun, L., Gounou, C., Marreddy, R.K., Picard, M., Broutin, I., Pos, K.M. and Lambert, O., (2016). Tripartite assembly of RND multidrug efflux pumps. Nature communications, 7, p.10731.
Du, D., van Veen, H.W., Murakami, S., Pos, K.M. and Luisi, B.F., (2015). Structure, mechanism and cooperation of bacterial multidrug transporters. Current opinion in structural biology, 33, pp.76-91.
Durán, N., Durán, M., de Jesus, M.B., Seabra, A.B., Fávaro, W.J. and Nakazato, G., (2016). Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine: Nanotechnology, Biology and Medicine, 12(3), pp.789-799.
Fitzpatrick, A.W., Llabrés, S., Neuberger, A., Blaza, J.N., Bai, X.C., Okada, U., Murakami, S., van Veen, H.W., Zachariae, U., Scheres, S.H. and Luisi, B.F., (2017). Structure of the MacAB–TolC ABC-type tripartite multidrug efflux pump. Nature microbiology, 2(7), p.17070.
Ling, L.L., Schneider, T., Peoples, A.J., Spoering, A.L., Engels, I., Conlon, B.P., Mueller, A., Schäberle, T.F., Hughes, D.E., Epstein, S. and Jones, M.,(2015). A new antibiotic kills pathogens without detectable resistance. Nature, 517(7535), p.455.
Melnyk, A.H., Wong, A. and Kassen, R., (2015). The fitness costs of antibiotic resistance mutations. Evolutionary applications, 8(3), pp.273-283.
Weatherspoon-Griffin, N., Yang, D., Kong, W., Hua, Z. and Shi, Y., (2014). The CpxR/CpxA two-component regulatory system upregulates the multidrug resistance cascade to facilitate Escherichia coli resistance to a model antimicrobial peptide. Journal of Biological Chemistry, pp.jbc-M114.
World Health Organisation (2018). Antimicrobial resistance Available at: http://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance [Accessed 12/07/2018]
Yamaguchi, A., Nakashima, R. and Sakurai, K., (2015). Structural basis of RND-type multidrug exporters. Frontiers in microbiology, 6, pp.302-327
Zgurskaya, H.I., Weeks, J.W., Ntreh, A.T., Nickels, L.M. and Wolloscheck, D., (2015). Mechanism of coupling drug transport reactions located in two different membranes. Frontiers in microbiology, 6, p.100.


