Enzymes Assignment Help
Enzymes are the catalyst that increases the rate of chemical reaction.
Chemical structure
Enzymes are protein hence, made up of chain of amino acid linked together by peptide bond. Therefore, the molecular weight ranges between 10,000 to 2, 000, 00. Beside this they are colloidal and thermolabile in nature along with specificity of their action. They have specificity in structure with a particular confirmation.
Role: Enzymes have several roles in the living body. It helps in breakdown of protein, fats and carbohydrates into smaller forms and many other enzymes helps in formation of large substances from the simpler ones. Like maltase hydrolyses maltose, proteases hydrolysis peptide linkage. Likewise, L amino acid oxidase act on L amino acid and exopeptidase hydrolysis terminals of protein chains, Urease acts on urea and so on. Therefore, enzymes have several functions in metabolic pathways and also they help in storage and release of energy. Along with this, they also helps in the process of reproduction, vision respiration etc.
Measurement of chemical activity: The chemical activity of an enzyme depends mainly on the concentration, temperature and the pH.The concentration of enzyme and substrate is important for all the chemical or enzymatic activities. With the increase in concentration of enzyme, velocity of the overall reaction also increases whereas increase in the concentration of substrate increases the velocity of enzyme. When enzyme interacts with the substrate, a product is obtained. Therefore the relationship between enzyme, velocity and substrate can be best explained mathematically with Michaelis- Meten constant and Lineweaver- Burk double reciprocal curve. In Michaelis- Meten constant, Km= [S] where km is Michaelis Meten constant that shows the use of substrate concentration for production of half- maximum velocity whereas low Km values indicated strong affinity between enzyme and substrate. Likewise, Lineweaver plot is used for finding Km value where, 1/v= Km/Vmax * 1/[S] +1/Vmax. Similarly, in case of temperature, with increase in temperature, kinetic energy increases and hence the rate of reaction increases. Therefore a bell shaped curve is obtained that givens the optimum temperature for maximum enzymatic activity. Above this temperature denaturation in structure of enzymes starts and hence making it inactive. Similar is the case of pH, higher and lower pH the activity of enzyme gets lowered. The enzyme activity is best at an optimum point when velocity is maximum.
Enzyme- substrate co-operation: It is very important that enzyme must bind to specific substrate for catalysis to take place. Therefore, different theories have been given that demonstrate the binding of enzyme with the substrate.
1. Fischers template theory: This theory is also called lock and key model and is given by German biochemist Emil Fischer. As per this theory, just like key fits into the lock, likewise the conformation of enzyme being rigid allows the specific substrate to fit at the active site. Since, this theory was not clearly explained and did not showed flexible nature of enzyme, therefore was not accepted.
2. Koshlands theory: This is also called induced fit theory given by koshland. According to this theory, when the substrate interacts with the enzymes it bring change in conformation that helps in catalysis i.e. enzyme are flexible and when particular substrate comes to it, the patter of enzyme changes forming a strong bond between enzyme and substrate.
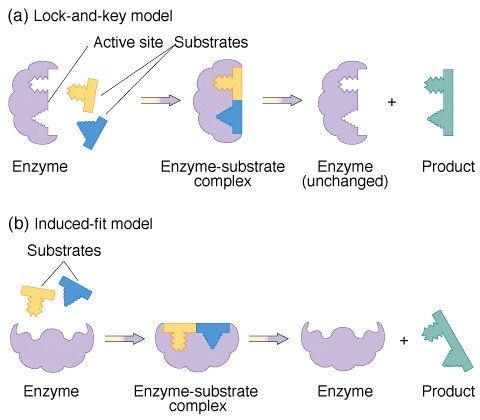
1. Fischers template theory: This theory is also called lock and key model and is given by German biochemist Emil Fischer. As per this theory, just like key fits into the lock, likewise the conformation of enzyme being rigid allows the specific substrate to fit at the active site. Since, this theory was not clearly explained and did not showed flexible nature of enzyme, therefore was not accepted.
2. Koshlands theory: This is also called induced fit theory given by koshland. According to this theory, when the substrate interacts with the enzymes it bring change in conformation that helps in catalysis i.e. enzyme are flexible and when particular substrate comes to it, the patter of enzyme changes forming a strong bond between enzyme and substrate.
Image reference: www.academic.brookly.cuny.edu
3. Substrate strain theory: According to this model, substrate is damaged due to the conformational change in the enzyme or the binding of substrate to the preformed active site induce damage to the substrate that in turn leads to product formation. Therefore, altogether the combination of second two models, i.e. Koshland and substrate strain theory given the actual information on the work and progress of enzyme for catalysis.
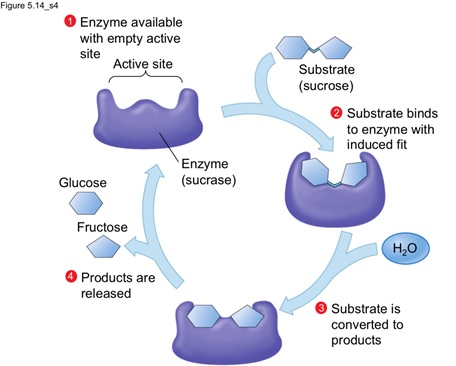
Image reference: www.studyblue.com
Coenzymes: The non- protein part of the enzyme that have low molecular weight are called coenzyme. These are the second substrate that undergo alteration during enzymatic reactions and have affinity for the enzyme that is comparable to the substrate. The enzyme that lacks its co- factor or co- enzyme is called holoenzyme and that have co- factor is called apoenzyme. The binding of co- enzyme with apoenzyme is called prosthetic group.
Inhibition: Inhibitor is the substance that binds with enzyme and decreases its catalytic activity. They can be categorized into three main groups:
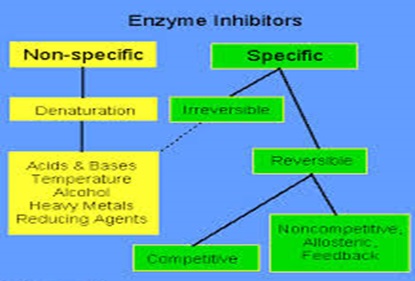
Image reference: www.elmhurst.edu
1. Reversible inhibition: the word reversible means the changes if made can be removed and hence bring everything back to its normal form. Likewise, this type of inhibitor binds to enzymes non- covalently inhibiting the reaction, but if removed enzyme inhibition gets reversed. This can be again divided into two main categories:
a. Competitive inhibition: In this case, the inhibitor resembles the real substrate and hence competes with it to bind at the active site of the enzyme. Therefore, till the time competitive inhibitor is bound to the active site of the enzyme, real substrate cannot bind to it and carry on the reaction. But if the concentration of substrate is increased then the reaction gets reversed and substrate can bind to the active site of the enzyme. Example includes succinate dehydrogenase whose substrate is succinic acid.
b. Non- competitive inhibition: In this case, the inhibitor does not bind to the active site and rather binds to the surface of the enzyme. Therefore, no any hurdle lies in binding of enzyme with substrate. But due to the change in confirmation by binding of inhibitor, catalysis does not take place. Example here includes binding of Ag+, Hg2+ etc. to particular enzyme.
2. Irreversible inhibition: In this case, once the inhibitor binds to the enzyme, and inactivates them. This type of binding result in toxic substance like that of DFP that bind to active site of serine forming DFP gas.
3. Allosteric inhibition: The non- covalent and reversible binding of effector molecule to the allosteric site also called inhibitor site that bring conformational change in the active site of enzyme is called allosteric inhibition.


