Chemistry Assignment Help With Solubility Product
- Home
- Chemistry
- Ionic Equilibrium
- Solubility Product

Solubility Product
It is the product of the molar concentrations of the ions in a saturated solution of an sparingly soluble salt with each concentration term raised to the power equal to the number of times that ion appears in balanced equation that represents equilibrium. It is denoted by Ksp.
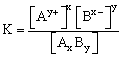
In saturated solution [AxBy] = constant
Applications of Solubility Product
(i) It helps in predicting the formation of a precipitate.
If ionic product > Ksp, precipitation occurs and if ionic product < Ksp no precipitate is formed.
(ii) Calculation of solubility of sparingly soluble salt - let solubility of salt AxBy in water at a particular temperature is S mole per litre. Then at equilibrium.
AxBy ![]()
![]()
e.g. for AgCl ![]()
![]()
Ksp = S2
Hence ![]()
ForAg2CrO4 ![]()
![]()
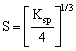
(iii) In qualitative analysis
The separation and identification of various basic radicals into different groups is based upon (a) solubility product principle and (b) common ion effect.
(iv) Purification of common salt.
Saturated solution of impure common salt is prepared and insoluble impurities are filtered off. HCl gas is passed through this solution. Thus, ionic product of ![]() exceeds the Ksp and pure NaCl precipitates out from the solution. This process is called salting out.
exceeds the Ksp and pure NaCl precipitates out from the solution. This process is called salting out.
Assignment Help For Solubility Product
Assignmenthelp.net provides best Online Assignment Help service in Solubility Product for all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Solubility Product assignment click here.
Chemistry Assignment Help | Chemistry Homework Help | Solubility Product Assignment Help | College Solubility Product Assignment Question Solutions | homework help | Homework Assignment Help | Online tutoring services | Term paper help | Online Tutoring


