Oxymercuration Demercuration Assignment Help

Oxymercuration Demercuration
In the overall Oxymercuration-Demercuration reaction, H2O is added to the double bond. The reaction is free from rearrangement (as it does not involve carbocation intermediate) and involves syn addition using Markownikov’s rule.
Alkenes react with mercuric acetate in the presence of water to give hydroxymercurial compounds which on reduction yield alcohols.
![]()
The first stage, oxymercuration involves addition to the carbon-carbon double bond of – OH and Hg(OAc)2. The electrophile of Hg(OAc)2 is AcOHg+ that adds C = C to form a similar to a bromonium ion. The mercurinium ion then reacts with H2O (not OAc) at the substituted carbon.
Oxymercuration Demercuration Assignment Help By Online Tutoring and Guided Sessions at AssignmentHelp.Net
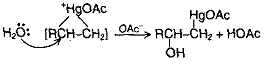
Then, in demercuration, HgOAc is replaced by H. The reaction sequence amounts to hydration of the alkene, but is much more widely applicable than direct acid catalyzed hydration.
Homework Help For Oxymercuration Demercuration
Assignmenthelp.net provides best Online Assignment Help service in Chemistry for all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Oxymercuration Demercuration assignment click here.


