Chemistry Assignment Help With Ionisation Of Water
Ionisation Of Water
Ionic product of water (
![]() )
)
Water is weak electrolyte, hence

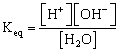
![]()
Since [H2O] = constant = ![]()
![]() depends on temperature. At 25° C value of
depends on temperature. At 25° C value of
![]() is 10–14.
is 10–14.
Hence ![]()
Thus ![]()
Or ![]()
Since [H+] = [OH–]
At 25°C, PH of pure water ![]()
Now at 25°C ![]()
or![]()
or
![]() at 25°C.
at 25°C.
Therefore PH range at 25° C will be 0 to 14
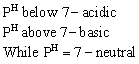

As temperature increases, degree of dissociation of water also increases, therefore value of ![]() increases.
increases.
Homework Help For Ionisation of Water
assignmenthelp.net provides best Online Assignment Help service in Ionisation of Water for all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Ionisation of Water assignment click here.


