Chemistry Homework Help With Halogenation

Halogenation
Chlorination may be brought about by photo irradiation, heat or catalysts and the extent of chlorination depends largely on the amount of chlorine used. A mixture of all possible isomeric monochlorides is obtained, but the isomers are formed in unequal amounts, due to difference in reactivity of primary, secondary; and tertiary hydrogen atoms.
The order of ease of substitution is
Tertiary Hydrogen > Secondary Hydrogen > Primary Hydrogen
Chlorination of iso butane at 25°C gives a mixture of two isomeric monochlorides.
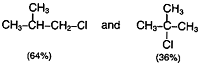
The tertiary hydrogen is replaced about 5 times as fast as primary hydrogen.
Bromination is similar to chlorination, but not so vigorous, lodination is reversible, but it may be carried out in the presence of an oxidising agent such as HIO3, HNO3 etc., which destroys they hydrogen iodide as it is formed and so drives the reaction to the right.
CH4 + I2 ® CH3I + HI
Iodides are more conveniently prepared by treating the chloro or bromo derivative with sodium iodide in methanol or acetone solution. For example,
RCl + NaI ![]() RI + NaCl
RI + NaCl
Direct fluorination is usually explosive. So special conditions are necessary for preparation of the fluorine derivatives of the alkanes.
RH + X2 ![]() RX + HX
RX + HX
Reactivity of X2 ;: F2 > Cl2 > Br2 > I2
Homework Help For Halogenation
assignmenthelp.net provides best Online Assignment Help service in Chemistry for all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Halogenation assignment click here.


