Chemistry Assignment Help With Elevation in Boiling Point

Elevation in Boiling Point
When a liquid is heated then due to rise in temperature the liquid vapourises and the vapour pressure increases. When the vapour pressure of the liquid becomes equal to the atmospheric pressure, the liquid begins to boil. The temperature at which the equilibrium vapour pressure of the liquid becomes equal to the atmospheric pressure is called boiling point.
When a non volatile solute is added to the solvent the vapour pressure of the solvent in the solution decreases. Consequently the solution has to be heated to a higher temperature to have its vapour pressure equal to atmospheric pressure.
Consider the graph of vapour pressure verses temperature in Kelvin scale.
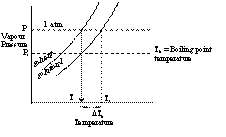
If T0 is the boiling point of solvent and T1 is the boiling point of solution, then
DTb = Elevation in boiling point
= T1 - T0
![]()
DTb = Kb × m
![]()
Kb = Molal elevation constant
Assignment Help For Elevation in Boiling Point
assignmenthelp.net provides best Online Assignment Help service in Elevation in Boiling Point for all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Elevation in Boiling Point assignment click here.


