ElectroChemistry Assignment Help
Introduction to Electrochemistry:
 The term Electrochemistry is reserved for the study of the processes that convert chemical energy to electrical energy and vice versa. Electrochemistry deals with the study of the chemical species and reactions that take place at the interface between an electron conductor and an ion conductor (electrolyte) in which an electron transfer occurs between the electrode and the electrolyte in solution.
The term Electrochemistry is reserved for the study of the processes that convert chemical energy to electrical energy and vice versa. Electrochemistry deals with the study of the chemical species and reactions that take place at the interface between an electron conductor and an ion conductor (electrolyte) in which an electron transfer occurs between the electrode and the electrolyte in solution.
Every type of Course Helps is available at assignmenthelp.net. We provide Assignment Help, project help, homework help, and online tutorial help for Electrochemistry. Our services are open for all students
Different aspects of the field of electrochemistry:
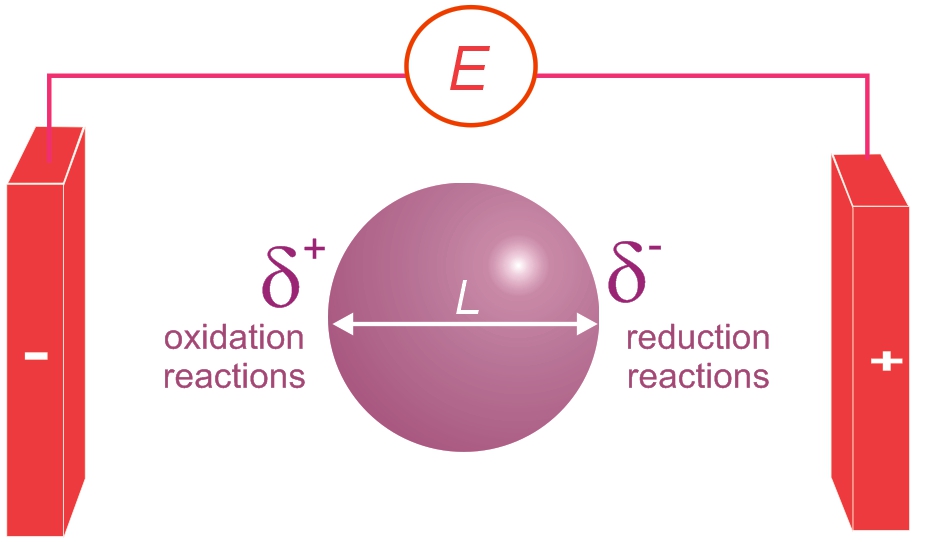
- Ionic: Concerns ions in solution and in the liquids arising from melting solids composed of ions.
- Electrodes: It concerns the region between an electronic and ionic conductor and the transfer of electric charge across it.
ElectroChemistry Assignment Help By Online Tutoring and Guided Sessions at AssignmentHelp.Net
Electrochemistry includes the following topics:
- Assigning oxidation numbers and review oxidation states.
- Redox Half-reactions
- Identify redox reactions
- Oxidizing agent
- Reducing agent
- Redox couples
- Balancing redox reactions
What kind of Assignment Help for Electrochemistry, We provide:
We provide all kind of help for help for Electrochemistry. If any student facing problem while doing their assignment and need help then they should visit assignmenthelp.net and chat with our expert and get solution for the problem. We also offer online tutorial for those who wish to learn help for Electrochemistry. All services are available for all students at a minimum cost. Anyone can join online tutorial and learn Electrochemistry in very less time.
Documentation for Electrochemistry is also available for quick reference. Students from college and school can also use all services available at assignmenthelp.net. Our all services are open for all students with minimum cost.
Email Based Assignment Help in Electrochemistry
To submit ElectroChemistry Course click here


