Chemistry Assignment Help With Dehydration Of Alcohols

Dehydration Of Alcohols
Alcohols when heated in presence of H2SO4, H3PO4, P2O5, Al2O3 or BF3 undergo loss of water molecule with the formation of alkene.
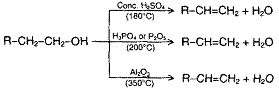
The reaction mechanism involves the following steps:
(i) In the first step OH group of the alcohol is protonated in a fast reversible reaction. Unlike OH group, protonated OH group is a good leaving group.
(ii) In the second step, water molecule is lost with the formation of a carbonium ion. This is the rate determining step.
(iii) In the final step carbonium ion loses proton from its adjacent carbon atom which results in more stable alkene. The anions of the acid or another alcohol molecule will function as a base and facilitate loss of proton.
Homework Help For Dehydration of Alcohols
assignmenthelp.net provides best Online Assignment Help service in chemistry for all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Dehydration of Alcohols assignment click here.


