Chemistry Assignment Help With Chemical Properties Of The Alkynes
Chemical Properties Of The Alkynes
The triple bond of alkynes consists of one sigma bond and two pie bonds, which are perpendicular to each other. The two pie bonds get mixed up and all the four pie electrons are cylindrical distributed around the two sp hybridised carbon atoms. Being unsaturated, alkynes undergo addition reactions to form alkene derivatives with the addition of one molecule and saturated compounds with the addition of two molecules. Under suitable conditions, it is possible to isolate the intermediate alkene.

Acetylene is less reactive than ethylene towards most of the electrophilic reagents. This is unexpected in view of the fact that the p-electron density in a triple bond is higher than that in a double bond. Possible reason for decreased reactivity of a triple bond towards electrophiles may be the fact that the bridged halonium ion from acetylene is more strained than the bridged halonium ion from ethylene.
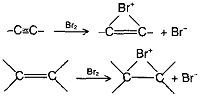
Towards hydrogenation (which do not involve electrophilic attack), ajkynes are more reactive than alkenes.
We are offering the following main topics for assignment like:
- Hydrogenation
- Addition Of Halogen Acids
- Addition Of Halogens
- Addition Of Water
- Addition Of Boron Hydrides
- Dimerisation
- Oxidation
- Ozonolysis
- Addition Of Hypohalous Acid
Homework Help For Chemical Properties of the Alkynes
Assignmenthelp.net provides best Online Assignment Help service in Alkanes for all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Chemical Properties of the Alkynes assignment click here


