Chemistry Assignment Help With Binary Non Ideal Solutions

Binary Non Ideal Solutions
Non-Ideal Solutions with Positive Deviation
Set-A solution shows a relationship where the vapour pressure of the solution observed is greater than the vapour pressure of solution under ideal conditions
i.e. Ps (obs) > Ps(actual)
or Ps > Pi where Pi =
![]() .....(8)
.....(8)
i.e. Ps >
![]() .....(9)
.....(9)
Such solutions are said to have a +ve deviation from ideality. The graph obtained resembles as given below
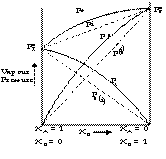
The reasons for such a +ve deviations could be linked to the fact that after mixing the interactions between the liquid A and B is less than the interaction between A–A system and B–B system.
i.e. A–B interactions < A–A interactions and B–B interactions.
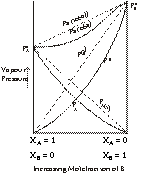
Non-Ideal Solution with Negative Deviation
The B set of solutions show a relationship where the vapour pressure of solution observed is lesser than the vapour pressure of the solution under ideal conditions i.e.
Ps (obs) < Psolution (ideal)
Ps < Pi.
Þ Ps < ![]()
A–B interaction > A–A interaction and B–B interactions.
Then the heat energy released when A–B interaction take place will be more than compared to the heat energy required for breaking the A–A interaction or B–B interactions. Hence the net result is
DHmix < 0 Þ DHmix = –ve and also
DVmix < 0 Þ DVmix = –ve
Assignment Help For Binary Non Ideal Solutions
Assignmenthelp.net provides best Online Assignment Help service in Binary Non Ideal Solutions or all standards. Our Tutor provide their high quality and optimized Tutorial help to fulfill all kind of need of Students.
To submit Binary Non Ideal Solutions assignment click here


